Accumulation of liquid hydrocarbons in catalyst pores during cobalt-catalyzed Fischer–Tropsch synthesis
Abstract
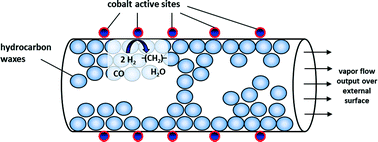
In the literature, it is widely claimed that during the initial period of Fischer–Tropsch synthesis liquid higher hydrocarbons fill catalyst pores completely, at least at temperatures of less than 250 °C at a typical pressure of about 2 MPa. This leads to diffusional restrictions in the porous network for particles with a size appropriate for industrial fixed-bed operation (>1 mm), whereby catalyst effectiveness and product selectivity are strongly affected. However, under industrially relevant reaction conditions, our experimental and theoretical investigations on the interplay of reaction and diffusion in cobalt catalyst particles reveal that the pores are only partly filled with liquid higher hydrocarbons even at a very long time on stream of months or more for a chain growth probability of below about 0.8. Experiments were conducted in a magnetic suspension balance using particles of so called “technical” size (in the regime of 1–3 mm, relevant for industrial application), and a mathematical model was developed describing quite accurately the formation, vaporization and accumulation of liquid products and their C-number distribution in the porous particle.

 Please wait while we load your content...
Please wait while we load your content...