Support effect for nanosized Au catalysts in hydrogen production from formic acid decomposition
Abstract
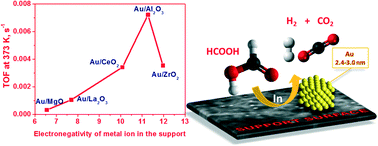
Catalysts with about 2.5 wt% of gold supported on Al2O3, ZrO2, CeO2, La2O3 and MgO oxides and with the same mean metal particle sizes of 2.4–3.0 nm have been studied in hydrogen production via formic acid decomposition. A strong volcano-type relation of the catalytic activity on the electronegativity of the support's cation was demonstrated with the Au/Al2O3 catalyst on the top. This indicated that the activity is affected by the acid–base properties of the support. A study of the most active Au/Al2O3 catalyst with aberration-corrected HAADF/STEM, XPS and EXAFS proved that gold is in metallic state. The content of single supported gold atoms/cations was negligible. Therefore, the mechanism of the reaction was related to the activation of formic acid on the catalyst's support followed by further decomposition of the formed reaction intermediate on the Au/support interface.
- This article is part of the themed collection: Nanocatalysis


 Please wait while we load your content...
Please wait while we load your content...