Effect of the Cα substitution on the ketonic decarboxylation of carboxylic acids over m-ZrO2: the role of entropy†
Abstract
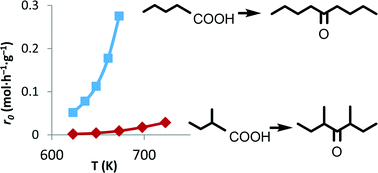
The kinetics of the ketonic decarboxylation of linear and branched carboxylic acids over m-ZrO2 as a catalyst has been investigated. The same apparent activation energy is experimentally determined for the ketonic decarboxylation of both linear pentanoic and branched 2-methyl butanoic acids, while the change in entropy for the rate-determining step differs by nearly 50 kJ mol−1. These results show that the difference in reactivity between linear and branched acids is due to entropic effects, and is related to the probability of finding the reactant molecules adsorbed and activated in a suitable way on the catalyst surface.

 Please wait while we load your content...
Please wait while we load your content...