Shape dependence of nanoceria on complete catalytic oxidation of o-xylene†
Abstract
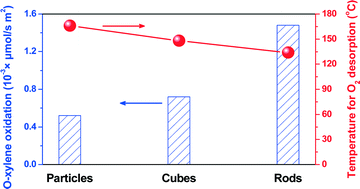
BTX (benzene, toluene, and xylene) in atmosphere, mainly emitted from various industrial processes and transportation activities, are of particular concern due to their potentially highly toxic effects on human health. Catalytic oxidation of o-xylene was investigated on nanosized CeO2 particles, cubes, and rods, among which rods show the highest activity, which is comparable with those of traditional noble-metal catalysts. CeO2 nanorods also exhibit long durability for o-xylene oxidation, without deactivation during a 50 h time-on stream test. Over the CeO2 rods and particles, the presence of water vapor slightly decreased o-xylene conversion, while water vapor enhanced o-xylene oxidation on the CeO2 cubes. High-resolution transmission electron microscopy, X-ray photoelectron spectroscopy, positron annihilation spectroscopy, and O2 temperature-programmed desorption measurements revealed that ceria rods enclosed by (111) and (100) facets exhibit the highest concentration of oxygen vacancy clusters (VCs), the presence of which promoted the adsorption of molecular oxygen. The lower the temperature for desorption of chemisorbed O2 species is, the higher is the activity for o-xylene oxidation, identifying the key role of VCs in this reaction via the activation of molecular oxygen over nanoceria. The finding may also be fundamental for designing ceria-based catalysts with better performance for catalytic oxidation of volatile organic compounds.

 Please wait while we load your content...
Please wait while we load your content...