Role of alkali earth metals over Pd/Al2O3 for decarbonylation of 5-hydroxymethylfurfural†
Abstract
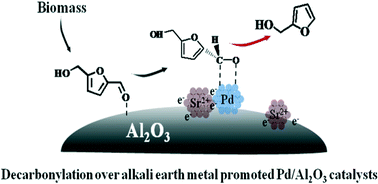
A series of Pd/Al2O3 catalysts with different alkali earth metals (Mg, Ca, Sr, Ba) and varying Sr loadings (1.8, 3.5, 5.3, 7 and 8.8 wt%) were investigated for 5-hydroxymethylfurfural (HMF) decarbonylation. The alkali earth metal and content were demonstrated to have profound influences on the metal dispersion, electron density of the metal, acid–base properties of the catalyst, and catalytic performance. The Pd3Sr/Al2O3 catalyst exhibited the highest initial activity and furfuryl alcohol selectivity, achieving a yield of 92%. The key to high decarbonylation selectivity is the suppression of hydrogenolysis and etherification side reactions through the attenuation of the acidity of catalysts. Successful catalytic activity not only lies in the increased metallic surface area, but is also affected by the adsorption properties of the carbonyl group and the poisoning CO produced. The catalytic activity is linearly correlated to the surface metallic area at low modifier loading over PdM/Al2O3 catalysts. But along with further increased metallic surface area over PdXSr/Al2O3, HMF conversion initially increased, reaching a plateau over Pd3Sr/Al2O3 and then decreased with increasing Sr loading. A synergistic effect between the Sr species and metallic Pd was proposed, which promoted the migration of carbonyl adsorption from the support to the surface Pd through the electron donation of Sr species to Al2O3 and metallic Pd.

 Please wait while we load your content...
Please wait while we load your content...