Enantioselective hydrogenation of ketones by iridium nanoparticles ligated with chiral secondary phosphine oxides†
Abstract
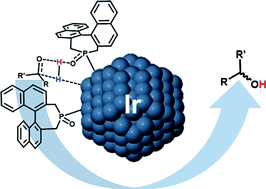
Chiral iridium nanoparticles (IrNPs) were synthesized by H2 reduction of (1,5-cyclooctadiene)(methoxy)iridium(I) dimer ([Ir(OMe)(COD)]2) in the presence of an asymmetric secondary phosphine oxide (4,5-dihydro-3H-dinaphtho[2,1-c:1′,2′-e] phosphepine-4-oxide, L). This highly reproducible and simple procedure furnished small, well dispersed and soluble nanoparticles of 1.4 (0.2) nm, which were found to be active catalysts for the enantioselective hydrogenation of prochiral ketones. This study represents the first example of asymmetric hydrogenation catalyzed by SPO-ligated metal nanoparticles and also the first example of asymmetric hydrogenation catalyzed by non-supported chiral IrNPs. The IrNPs were characterized by the use of a wide variety of techniques, such as TEM, HRTEM, EDX, XPS, ATR FT-IR, ECD, and MAS-NMR spectroscopy with and without 1H–13C cross-polarization (CP).

 Please wait while we load your content...
Please wait while we load your content...