Mechanistic insights into selective hydrodeoxygenation of lignin-derived β-O-4 linkage to aromatic hydrocarbons in water†
Abstract
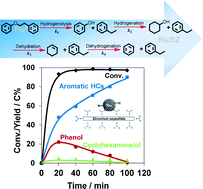
The route for selective hydrodeoxygenation of phenethoxybenzene (PEB, which represents the dominant β-O-4 linkage in lignin) to produce benzene and ethylbenzene is realized by employing a multi-functional Ru/sulfate zirconium (Ru/SZ) catalyst in the aqueous phase. One-pot hydrodeoxygenation of PEB is initially cleaved, forming C6 phenol and C8 ethylbenzene via the selective cleavage route of the Caliphatic–O bond (k1). While the C8 ethylbenzene is stable against further hydrogenation under the selected conditions, the C6 phenol intermediate is hydrogenated to cyclohexanol with a rapid rate (k2), and the sequential steps of cyclohexanol dehydration (k3) as well as cyclohexene dehydrogenation (k4) lead to the target benzene formation. A high temperature (240 °C) together with a low hydrogen pressure (8 bar) is essential for achieving such a specific route, suppressing the side-reactions of aromatic hydrogenation. Compared to Ru/SZ, Pd/SZ catalyzes the high rate for cleavage of ether (k1), but fails in sequential C6 phenol hydrogenation (k2) and cyclohexanol dehydration (k3), and thus is not able to accomplish the complex cascade reaction. Pt/SZ performs poorly in the primary step of ether cleavage (k1), and therefore, blocks the following sequential steps. The separate kinetics tests on phenol and cyclohexanol hydrodeoxygenation reveal that benzene is not produced by direct hydrogenolysis, but by the dehydration–dehydrogenation route. The high benzene yield from phenol is probably attributed to few surface adsorbed H˙ species retained on Ru/SZ during phenol hydrodeoxygenation, as evidenced by the model reaction of cyclooctene hydrogenation with the used Ru/SZ in the presence of N2.

 Please wait while we load your content...
Please wait while we load your content...