Identification of the reaction pathway and reactive species for the selective catalytic reduction of NO with NH3 over cerium–niobium oxide catalysts†
Abstract
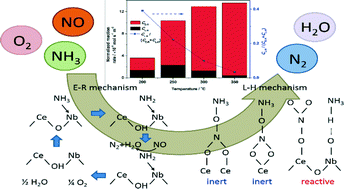
A series of Ce–Nb oxide catalysts were synthesized at different calcination temperatures for the selective catalytic reduction (SCR) of NO with NH3 and the reaction pathway and reactive species were investigated in detail. The SCR reaction pathway over the Ce–Nb catalysts followed both the Eley–Rideal mechanism and the Langmuir–Hinshelwood mechanism, where the former contributed more especially at high reaction temperatures. Lewis acidity was found to be catalytically important in the Eley–Rideal mechanism. NO2 and monodentate nitrate were the main reactive species in the Langmuir–Hinshelwood mechanism. The catalyst calcined at lower temperatures exhibited higher catalytic activity at low temperatures but lower activity at high temperatures. With the increase in calcination temperature, the catalyst surface was gradually covered with niobium oxide species, resulting in the enhancement of total acidity but the decline of redox ability, along with the decrease in the contribution of the Langmuir–Hinshelwood mechanism to the SCR reaction.

 Please wait while we load your content...
Please wait while we load your content...