The performance and mechanism of Ag-doped CeO2/TiO2 catalysts in the catalytic oxidation of gaseous elemental mercury
Abstract
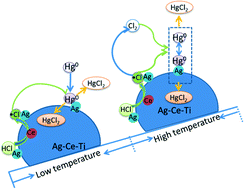
To improve the ability of CeO2/TiO2 catalysts to catalyze the oxidation of gaseous elemental mercury, silver was introduced. Doping with Ag can significantly enhance the Hg0 oxidation ability of CeO2/TiO2. In addition, the temperature window was widened (from 150 to 450 °C). The catalysts were characterized by TEM, XRD, XPS and H2-TPR. The results indicated that silver nanoparticles can be loaded on the TiO2 support. The catalysts had better crystallization and higher redox ability after addition of silver. Silver existed mostly in its metallic state, which can keep Ce in a higher Ce(IV) state. HCl was oxidized into active Cl by CeO2 and then was adsorbed on the silver nanoparticles. In addition to the HCl and Hg0 breakthrough experiments, a Hg0 desorption experiment and a Cl2 yield experiment were conducted to study the catalytic mechanisms of elemental mercury oxidation over various temperature ranges; these experiments indicated that the reaction followed the Langmuir–Hinshelwood mechanism at low temperature, and the Eley–Rideal mechanism and homogeneous gas-phase reaction at high temperature. Furthermore, a mercury valence state change experiment was performed, which indicated that HCl was the major catalytic oxidization component.

 Please wait while we load your content...
Please wait while we load your content...