Direct synthesis of dimethyl ether from CO2 hydrogenation over Cu–ZnO–ZrO2/SO42−–ZrO2 hybrid catalysts: effects of sulfur-to-zirconia ratios†
Abstract
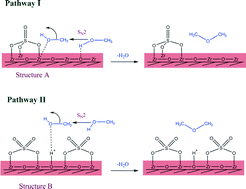
Sulfated zirconia catalysts were prepared by a direct sulfation method and were admixed with a CuO–ZnO–ZrO2 catalyst for the direct synthesis of DME from CO2 hydrogenation. The effects of sulfur-to-zirconia ratios on the physicochemical properties, activity, selectivity and stability of the catalysts were investigated. The sulfur loading content significantly influenced the structure and surface chemistry of the catalysts. The addition of a small amount of sulfur (5–15 wt%) created numerous mesopores on the catalyst surface, remarkably enhancing the surface area and total pore volume. However, at high sulfur loading (20–30 wt%), the mesopores tended to merge and form a larger pore. The detailed characterization by FT-IR, XANES and NH3-TPD reveals that the sulfated zirconia with low sulfur content (5–10 wt%) mainly contained weak acid sites and acted as Lewis acids. Increasing the sulfur loading (15–30 wt%) resulted in the formation of Brønsted acid sites, thus increasing the acid strengths. The sulfated zirconia catalyst with 20 wt% sulfur loading achieved a superior DME productivity of 236 gDME kgcat−1 h−1 at a reaction temperature and pressure of 260 °C and 20 MPa. However, after 75 h of a time-on-stream experiment, the sulfated zirconia catalyst lost approximately 16.9% of its initial activity while a commercial H-ZSM-5 catalyst was more stable as only a 2.85% reduction was observed.

 Please wait while we load your content...
Please wait while we load your content...