Impact of particle size and metal–support interaction on denitration behavior of well-defined Pt–Cu nanoparticles
Abstract
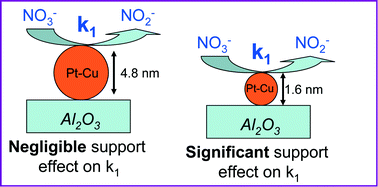
Well-dispersed Pt–Cu nanoparticles with two average sizes were synthesized: small (≈1.6 nm) and large (≈4.8 nm), respectively. The effects of size and support on the catalytic behavior of the nanoparticles for denitration reaction were analyzed. Both the unsupported and alumina-supported nanoparticles of smaller average size (1.6 nm) were very active, completely converting NO3− and NO2− (an intermediate reaction product) to N2 and NH4+. They were also more selective of N2 compared to the larger particles. The effects of particle size and alumina support on the denitration rate constants were analyzed in detail. The kinetic investigation indicates that the catalytic behavior is strongly related to the size of Pt–Cu nanoparticles as well as to the metal–support interaction. Generally, the larger nanoparticles proved to be less active, but on the other hand, they are less influenced by the support than the smaller ones. The metal–support interaction for bimetallic nanoparticles with smaller average size proved to be a key factor both for nitrate and nitrite reduction. Consideration of the reaction mechanism is made in light of the experimental results.

 Please wait while we load your content...
Please wait while we load your content...