An in situ acidic carbon dioxide/glycol system for aerobic oxidative iodination of electron-rich aromatics catalyzed by Fe(NO3)3·9H2O†
Abstract
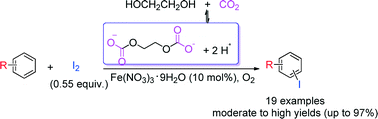
An environmentally benign CO2/glycol reversible acidic system was developed for the iron(III)-catalyzed aerobic oxidative iodination of electron-rich aromatics without the need for any conventional acid additive or organic solvent. Notably, moderate to high isolated yields (up to 97%) of the aryl iodides were attained with comparable regioselectivity when ferric nitrate nonahydrate was used as the catalyst with molecular iodine under 1 MPa of CO2.

 Please wait while we load your content...
Please wait while we load your content...