Mechanistic analysis of water oxidation catalyzed by mononuclear copper in aqueous bicarbonate solutions†
Abstract
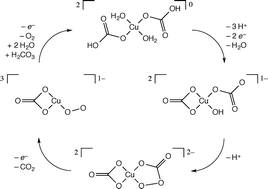
We characterize a mechanism for a monomeric copper catalyst reported to oxidize water in bicarbonate solution when subject to sufficiently high external potentials at near neutral pH values. Density functional computations establish the thermochemical equilibria associated with microscopic redox and proton transfer steps and further reveal that O–O bond formation is associated with the unusual reaction of a coordinated hydroxide and carbonate ligand to generate a peroxycarbonate intermediate. The peroxycarbonate complex then decomposes through a retrocyclization to liberate O2 and CO2 and ultimately complete the catalytic cycle.

 Please wait while we load your content...
Please wait while we load your content...