Catalytic consequences of micropore topology, mesoporosity, and acidity on the hydrolysis of sucrose over zeolite catalysts†
Abstract
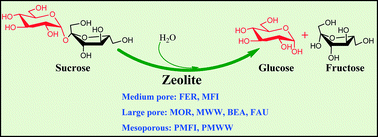
The effects of zeolite micropore topology, mesoporosity, and acidity on the hydrolysis of polysaccharides were probed in reactions of sucrose over a variety of zeolite catalysts (FER, MFI, MOR, MWW, BEA, FAU, pillared MFI (PMFI), and pillared MWW (PMWW)). The measured rate of sucrose hydrolysis over microporous zeolites varied by a factor of ~100 following a trend of FER < MOR ~ MFI < BEA ~ MWW < FAU, indicating that the hydrolysis of sucrose increases with increasing zeolite micropore sizes. The presence of mesoporosity in PMFI and PMWW zeolites enhanced the rates of sucrose reactions by a factor of ~2 in comparison with the microporous MWW and MFI zeolites, which may result from the enhanced acid site accessibility and mitigated diffusion constraints. The examination on the effects of zeolite acidity on the hydrolysis of sucrose by employing MFI, PMFI, BEA, MWW, and FAU zeolites with a range of Si/Al ratios showed that a Si/Al ratio of ~70–150 provides a maximal rate constant per acid site in the catalysts. The measured activation energies of the catalytic reactions in all zeolites were similar. The measured entropies, however, increased abruptly with increasing micropore sizes of zeolites and slightly with increasing mesoporosity in the zeolites. The present study suggests that the hydrolysis of sucrose is driven primarily by the reaction entropies that are dominated consecutively by the micropore topology, acidity, and mesoporosity of the zeolite catalysts.

 Please wait while we load your content...
Please wait while we load your content...