One-pot synthesis of furfural derivatives from pentoses using solid acid and base catalysts†
Abstract
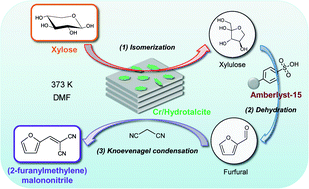
One-pot synthesis of (2-furanylmethylene)malononitrile, a Knoevenagel product of furfural with malononitrile, from xylose efficiently proceeded by combined use of acid Amberlyst-15 and acid-base Cr/hydrotalcites in 44% yield. Structural characterization was carried out using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy, and nitrogen adsorption measurements. The Lewis acidic properties of the highly active Cr/HT were investigated using the Meerwein–Ponndorf–Verley (MPV) reaction. It was confirmed that the prepared Cr/HT possessed the Lewis acid Cr2O3 on the HT surface. Thus, combined use of the dispersed Lewis acid Cr2O3 and the Brønsted base HT facilitated the isomerization step of aldose into ketose and strongly promoted activity for the synthesis of furfural derivatives from aldoses through isomerization, dehydration and Knoevenagel condensation reactions in a one-pot manner.

 Please wait while we load your content...
Please wait while we load your content...