Pyrochlore-like K2Ta2O6 synthesized from different methods as efficient photocatalysts for water splitting†
Abstract
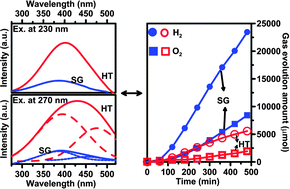
Pyrochlore-type K2Ta2O6 with a high dielectric constant, which may lead to facile charge separation, is a prospective medium for photocatalytic
- This article is part of the themed collection: Photocatalysis

 Please wait while we load your content...
Please wait while we load your content...