Kinetics of propane dehydrogenation over Pt–Sn/Al2O3†
Abstract
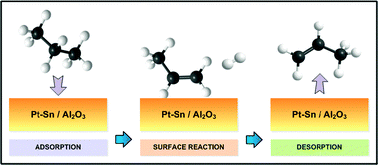
The kinetics of the gas-phase dehydrogenation of propane over Pt–Sn (1 : 1 mol ratio) supported on Al2O3 was investigated for the first time over the high range of reactant/products partial pressures (up to 0.875 atm). The Pt precursor was reduced to metallic form after a temperature-programmed reduction (TPR) at 873 K. X-ray photoelectron spectroscopy (XPS) analysis suggests that a Pt–Sn surface alloy forms, decreasing the H2 adsorption on the Pt–Sn sites (5.6 nm in average size) relative to monometallic Pt (6.8 nm). We performed kinetic studies in the absence of mass/heat transfer limitations. The incorporation of Sn into Pt in Pt–Sn/Al2O3 enhanced the catalytic activity and stability when compared to Pt/Al2O3. We attribute this response to the surface electronic interaction between Pt and Sn. The initial propane consumption rate increases with the partial pressure of propane and decreases with the partial pressure of propene, while varying that of hydrogen has a negligible effect. Applying the Langmuir–Hinshelwood–Hougen–Watson (LHHW) approach, the most likely kinetic model is non-dissociative adsorption of propane with simultaneous release of H2, where surface reaction is the rate-limiting step. Our results of the kinetic aspects provide practical insights relevant to propane dehydrogenation.

 Please wait while we load your content...
Please wait while we load your content...