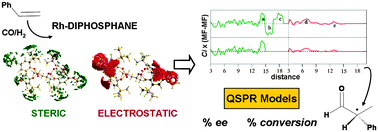
This paper describes the development of quantitative structure–enantioselectivity and–activity relationships for the asymmetric hydroformylation of styrene by Rh–diphosphane. We used 3D steric- and electrostatic-type interaction fields derived from DFT calculations, and generated alignment-independent descriptors using GRid INdependent Descriptor (GRIND) methodology. The obtained QSPR models showed statistical significance and predictive ability. The most predictive model for enantioselectivity was obtained using steric-type 3D fields (MSF) based on the local curvature electron density isosurface that accounts for catalyst shape (r2 = 0.92, q2 = 0.68). We obtained the most predictive model for activity using a combination of shape- and electrostatic-based 3D fields (r2 = 0.99, q2 = 0.74). The use of chemically meaningful descriptors provides insight into the factors governing catalytic activity and selectivity. The worst predicted ligand, kelliphite, showed the lowest preference for equatorial–apical coordination and low selectivity, which suggests that its intrinsic enantiotopic differentiation capacity can be lost through the occurrence of bis-equatorial paths. The selective catalysts Rh–chiraphite, –binapine, –diazaphospholane, and –yanphos showed the same pattern as Rh–binaphos, for which the origin of stereoinduction is known: the chirality at the apical site discriminates one alkene coordination path and one enantiomer. Ligands with electron withdrawing groups at phosphorus atoms such as chiraphite, kelliphite, binaphos, and diazaphospholane reduce ligand basicity and promote catalytic activity effectively. However, more complex relationships underlie the origin of activity, and the shape of the catalyst also needs to be considered such as for the Ph-BPE ligand. Comparison with previous studies suggests that reduction of the steric hindrance at the reaction centre which favors alkene coordination and insertion would also promote catalytic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...