Selective oxidation of benzyl alcohol by two phase electrolysis using nitrate as mediator
Abstract
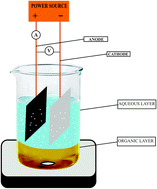
A convenient, selective and inexpensive two phase electrolysis method has been developed for preparation of benzaldehyde. Optimization studies have been carried out on an undivided beaker-type cell equipped with carbon anode and stainless steel cathode using a two-phase electrolytic medium made up of an aqueous solution of sodium nitrate with a minimum amount of HCl as supporting electrolyte and chloroform containing benzyl alcohol as organic phase at 30–34 °C. In order to oxidize one mole of benzyl alcohol to benzaldehyde 2 F of electricity was passed. Two-phase electrolysis results in high yield (95%) and high current efficiency (95%).

 Please wait while we load your content...
Please wait while we load your content...