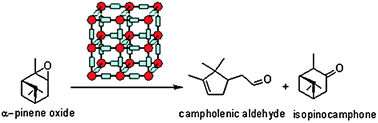
A series of Fe3+-containing porous metal–organic frameworks (MOFs), including the commercial iron trimesate Basolite F-300 or Fe(BTC) (BTC: 1,3,5-benzenetricarboxylate) and the synthetic iron terephthalate MIL-88B (Fe3O(BDC)3X, X = Cl, OH, BDC = 1,4-benzenedicarboxylate), iron naphthalenedicarboxylate MIL-88C (Fe3O(NDC)3X, X = Cl, OH, NDC = 1,6-naphthalenedicarboxylate), iron trimesate MIL-100 (Fe3O(BTC)2X, X = Cl, OH) and iron azobenzenetetracarboxylate soc-MOF(Fe) or MIL-127 (Fe6O2(Tazb)3X2, X = Cl, OH, Tazb = 3,3′,5,5′-azobenzenetetracarboxylate; MIL stands for Materials from Institut Lavoisier), have been tested for the rearrangement of α-pinene oxide to camphonelal and isopinocamphone in the absence of solvent. Conversions of about 10% with 50% selectivity towards camphonelal were obtained. This catalytic performance has been compared with that of the copper trimesate Basolite C-300 or HKUST-1 of formula Cu3(BTC)2 and the aluminium terephthalate Basolite A-100 or MIL-53(Al) of formula Al(OH)(BDC) as well as with some homogeneous (ZnCl2, Cu(NO3)2, Al(NO3)3) and heterogeneous (Fe3+-exchanged Y-zeolite) Lewis acids. Fe(BTC) also exhibits catalytic activity for the rearrangement of other epoxides (styrene, cyclohexene and norbornene oxides) under solventless conditions. Some partial deactivation of the Fe(BTC) with a slight degradation of the structure has nevertheless been observed.

 Please wait while we load your content...
Please wait while we load your content...