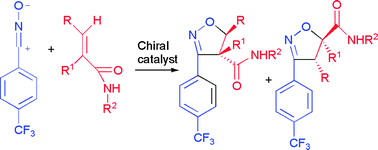
Chiral Lewis acid mediated 1,3-dipolar cycloaddition reactions of aryl nitrile oxides and secondary α- or β-substituted acrylamides and cinnamides were examined. Excellent enantioselectivities with moderate to good regioselectivities were achieved for crotonamides with complexes of carbohydrates with Yb(OTf)3, TiCl4, Mg(OTf)2, and CsF as well as with the (−)-sparteine–Yb(OTf)3 system. High enantiomeric excess and high regioselectivity were observed for cinnamides in reactions mediated by Yb(OTf)3 complexes with carbohydrate, R-BINOL, and (−)-sparteine.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...