The recent achievements of redox-neutral radical C–C cross-coupling enabled by visible-light
Abstract
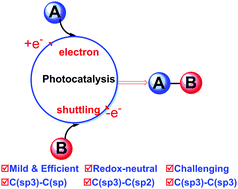
Visible-light photoredox catalysis has been esteemed as one sustainable and attractive synthetic tool. In the past four years, a new yet challenging trend, visible-light-driven redox-neutral radical C–C cross-coupling involving putative radical intermediates, has been booming rapidly. Its advent brings a powerful platform to achieve non-classical C–C connections, and should lead to fundamental changes in retrosynthetic analysis. In this tutorial review, we highlight the recent achievements of visible-light-mediated redox-neutral radical C(sp3)–C(sp2), C(sp3)–C(sp), and C(sp3)–C(sp3) bond formation, opening a new window for C–C cross-coupling through the photoredox electron shuttling cycle between two coupling partners. While radical–radical coupling steered by the persistent radical effect was proposed as a rational explanation for the redox-neutral photoredox events, alternative kinetically driven chain propagation and radical addition pathways cannot be ruled out. This tutorial review aims to highlight the recent achievements of photoredox-neutral radical C–C coupling in synthetic chemistry.


 Please wait while we load your content...
Please wait while we load your content...