Organocatalytic Lewis base functionalisation of carboxylic acids, esters and anhydrides via C1-ammonium or azolium enolates
Abstract
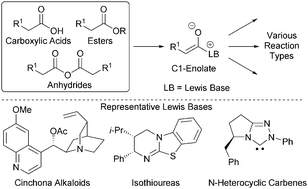
This tutorial review highlights the organocatalytic Lewis base functionalisation of carboxylic acids, esters and anhydrides via C1-ammonium/azolium enolates. The generation and synthetic utility of these powerful intermediates is highlighted through their application in various methodologies including aldol-lactonisations, Michael-lactonisations/lactamisations and [2,3]-rearrangements.

 Please wait while we load your content...
Please wait while we load your content...