Gold-catalyzed C(sp3)–H bond functionalization
Abstract
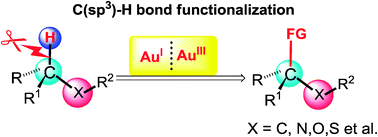
C–H bonds are ubiquitous in organic molecules. Homogenous gold-catalyzed direct functionalization of unsaturated C–H bonds has emerged as a powerful method in our synthetic toolbox. However, Csp3–H bonds have larger dissociation energy and lower proton acidity, and thus the efficient and exquisitely selective cleavage of this kind of chemical bonds for the formation of new carbon–carbon and carbon–heteroatom bonds is still a great challenge. In this tutorial review, we will highlight the recent achievements of gold-catalyzed oxidative and redox–neutral Csp3–H bond functionalization, which opens new avenues for economical and sustainable construction of fine chemicals.

 Please wait while we load your content...
Please wait while we load your content...