Enantioselective methodologies using N-carbamoyl-imines
Abstract
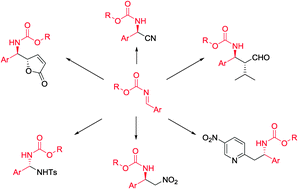
Nucleophilic addition to carbon–nitrogen double bonds (imines) represents one of the most common strategies for the synthesis of amine derivatives. In order to circumvent the problem associated with low reactivity of imines in nucleophilic addition, various imines with electron-withdrawing groups at nitrogen have been studied, and many of them were successfully applied in asymmetric methodologies. Especially N-carbamoyl imines were found to be useful in the enantioselective synthesis of various organic compounds, due to their increased reactivity toward nucleophiles as well as limited difficulties connected with the removal of the carbamoyl moiety in target molecules. The aim of this review is to cover enantioselective methods based on N-carbamoyl imines, focusing on synthetically useful protocols.

 Please wait while we load your content...
Please wait while we load your content...