Crystallization under nanoscale confinement
Abstract
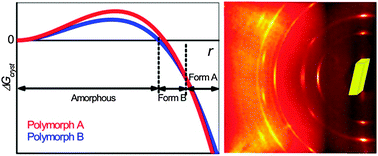
Classical crystal growth models posit that crystallization outcomes are determined by nuclei that resemble mature crystal phases, but at a critical size where the volume free energy of nuclei begins to offset the unfavorable surface free energy arising from the interface with the growth medium. Crystallization under nanoscale confinement offers an opportunity to examine nucleation and phase transformations at length scales corresponding to the critical size, at which kinetics and thermodynamics of nucleation and growth intersect and dramatic departures in stability compared to bulk crystals can appear. This tutorial review focuses on recent investigations of the crystallization of organic compounds in nanoporous matrices that effectively provide millions of nanoscale reactors in a single sample, ranging from controlled porous glass (CPG) beads to nanoporous block-copolymer monoliths to anodic aluminum oxide (AAO) membranes. Confinement of crystal growth in this manner provides a snapshot of the earliest stages of crystal growth, with insights into nucleation, size-dependent polymorphism, and thermotropic behavior of nanoscale crystals. Moreover, these matrices can be used to screen for crystal polymorphs and assess their stability as nanocrystals. The well-aligned cylindrical nanoscale pores of polymer monoliths or AAO also allow determination of preferred orientation of embedded nanocrystals, affording insight into the competitive nature of nucleation, critical sizes, and phase transition mechanisms. Collectively, these investigations have increased our understanding of crystallization at length scales that are deterministic while suggesting strategies for controlling crystallization outcomes.
- This article is part of the themed collection: Nucleation and crystallisation

 Please wait while we load your content...
Please wait while we load your content...