Physical studies of heterometallic rings: an ideal system for studying magnetically-coupled systems†‡
Abstract
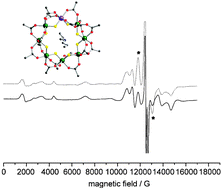
Heterometallic rings of general formula [Cat][M7M′F8(O2CtBu)8] (M = a trivalent metal, M′ = a divalent metal, cat = a secondary ammonium
- This article is part of the themed collection: Alfred Werner Nobel Prize 100 year celebration

 Please wait while we load your content...
Please wait while we load your content...