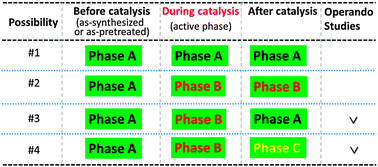
Bimetallic catalysts are one of the main categories of metal catalysts due to the tunability of electronic and geometric structures through alloying a second metal. The integration of a second metal creates a vast number of possibilities for varying the surface structure and composition of metal catalysts toward designing new catalysts. It is well acknowledged that the surface composition, atomic arrangement, and electronic state of bimetallic catalysts could be different from those before a chemical reaction or catalysis based on ex situ studies. Thanks to advances in electron-based surface analytical techniques, the surface chemistry and structure of bimetallic nanoparticles can be characterized under reaction conditions and during catalysis using ambient pressure analytical techniques including ambient pressure XPS, ambient pressure STM, X-ray absorption spectroscopy and others. These ambient pressure studies revealed various restructurings in the composition and arrangement of atoms in the surface region of catalysts under reaction conditions or during catalysis compared to that before reaction. These restructurings are driven by thermodynamic and kinetic factors. The surface energy of the constituent metals and adsorption energy of reactant molecules or dissociated species on a metal component are two main factors from the point of view of thermodynamics. Correlations between the authentic surface structure and chemistry of catalysts during catalysis and simultaneous catalytic performance were built for understanding catalytic mechanisms of bimetallic catalysts toward designing new catalysts with high activity, selectivity, and durability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...