Kinetics, products, and mechanisms of secondary organic aerosol formation†
Abstract
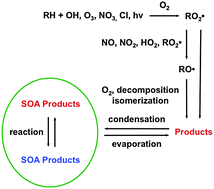
Secondary organic aerosol (SOA) is formed in the atmosphere when volatile organic compounds (VOCs) emitted from anthropogenic and biogenic sources are oxidized by reactions with OH radicals, O3, NO3 radicals, or Cl atoms to form less volatile products that subsequently partition into aerosol particles. Once in particles, these organic compounds can undergo heterogenous/multiphase reactions to form more highly oxidized or oligomeric products. SOA comprises a large fraction of atmospheric aerosol mass and can have significant effects on atmospheric chemistry, visibility, human health, and climate. Previous articles have reviewed the kinetics, products, and mechanisms of atmospheric VOC reactions and the general chemistry and physics involved in SOA formation. In this article we present a detailed review of VOC and heterogeneous/multiphase chemistry as they apply to SOA formation, with a focus on the effects of VOC molecular structure on the kinetics of initial reactions with the major atmospheric oxidants, the subsequent reactions of alkyl, alkyl peroxy, and alkoxy radical intermediates, and the composition of the resulting products. Structural features of reactants and products discussed include compound carbon number; linear, branched, and cyclic configurations; the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and aromatic rings; and functional groups such as carbonyl, hydroxyl, ester, hydroxperoxy, carboxyl, peroxycarboxyl, nitrate, and peroxynitrate. The intention of this review is to provide atmospheric chemists with sufficient information to understand the dominant pathways by which the major classes of atmospheric VOCs react to form SOA products, and the further reactions of these products in particles. This will allow reasonable predictions to be made, based on molecular structure, about the kinetics, products, and mechanisms of VOC and heterogeneous/multiphase reactions, including the effects of important variables such as VOC, oxidant, and NOx concentrations as well as temperature, humidity, and particle acidity. Such knowledge should be useful for interpreting the results of laboratory and field studies and for developing atmospheric chemistry models. A number of recommendations for future research are also presented.
C bonds and aromatic rings; and functional groups such as carbonyl, hydroxyl, ester, hydroxperoxy, carboxyl, peroxycarboxyl, nitrate, and peroxynitrate. The intention of this review is to provide atmospheric chemists with sufficient information to understand the dominant pathways by which the major classes of atmospheric VOCs react to form SOA products, and the further reactions of these products in particles. This will allow reasonable predictions to be made, based on molecular structure, about the kinetics, products, and mechanisms of VOC and heterogeneous/multiphase reactions, including the effects of important variables such as VOC, oxidant, and NOx concentrations as well as temperature, humidity, and particle acidity. Such knowledge should be useful for interpreting the results of laboratory and field studies and for developing atmospheric chemistry models. A number of recommendations for future research are also presented.
- This article is part of the themed collection: Atmospheric chemistry

 Please wait while we load your content...
Please wait while we load your content...