Disentangling interfacial redox processes of proteins by SERR spectroscopy
Abstract
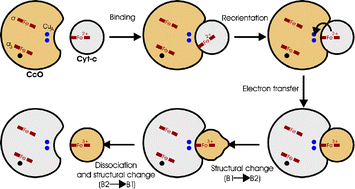
Surface-enhanced resonance-Raman spectroelectrochemistry represents a powerful approach for studying the structure and reaction dynamics of redox
- This article is part of the themed collection: Surface-enhanced Raman Spectroscopy

 Please wait while we load your content...
Please wait while we load your content...