Iodine electrophiles in stereoselective reactions: recent developments and synthetic applications
Abstract
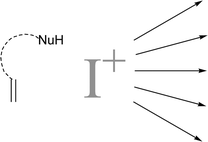
The regio- and stereocontrolled functionalisation of carbon–carbon double bonds bears enormous potential in organic synthesis. This area has been extensively studied and reviewed as

 Please wait while we load your content...
Please wait while we load your content...