Non-stabilized transition metal carbenes as intermediates in intramolecular reactions of alkynes with alkenes
Abstract
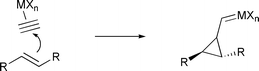
In this tutorial review we summarize the two major pathways followed in the reaction of
- This article is part of the themed collection: Celebrating the ChemSocRev Lectureship winners

 Please wait while we load your content...
Please wait while we load your content...