Predicting structural and energetic changes in Met–aromatic motifs on methionine oxidation to the sulfoxide and sulfone†
Abstract
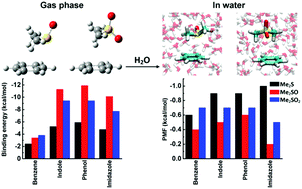
Noncovalent interactions between Met and aromatic residues define a common Met–aromatic motif in proteins. Met oxidation to MetOn (n = 1 sulfoxide, n = 2 sulfone) alters protein stability and function. To predict the chemical and physical consequences of such oxidations, we modeled the chemistry and redox properties of MetOn–aromatic complexes in depth for comparison with our Met–aromatic models (E. A. Orabi and A. M. English, J. Phys. Chem. B, 2018, 122, 3760). We describe here ab initio quantum mechanical calculations at the MP2(full)/6-311++G(d,p) level of theory on complexes of MetOn (n = 1, 2; modeled by Me2SO and Me2SO2) with models of the side-chains of Phe (benzene, toluene), Trp (indole, 3-methylindole), Tyr (phenol, 4-methylphenol) and His (imidazole, 4-methylimidazole). Binding energies of the global minimum conformers (−3.4 to −11.9 kcal mol−1) indicate that the gas-phase Me2SOn–aromatics are 40–115% more stable than the Me2S–aromatics. Binding of S between the edge and face of the aromatic ring is favored in most complexes as it accommodates both robust σ- and π-type H-bonding. Interactions involving the σ-holes on the S atoms (σ-hole⋯πar and σ-hole⋯Nar/Oar), as well as S⋯π interactions in the sulfoxides, contribute to complex stability. Complexation modulates the ionization potential (IP) of the interacting fragments with the binding geometry dictating the center oxidized in the Me2SO-aromatics whereas the aromatic is oxidized in the Me2SO2 complexes because of the sulfone's high IP. Potentials of mean force reveal binding free energies of −0.2 to −0.7 kcal mol−1 in bulk water, which indicates that the Me2SOn–aromatics are up to 80% less stable than the corresponding aqueous Me2S–aromatics. Molecular dynamics simulations predict that Me2SOn preferentially interacts with the ring face and expose the dominance of π- vs. σ-type H-bonding in the hydrated complexes as found for the Me2S–aromatics. Our modeling will inform how Met/MetOn–aromatic motifs are determinants of redox-induced changes in proteins.


 Please wait while we load your content...
Please wait while we load your content...