From in silica to in silico: retention thermodynamics at solid–liquid interfaces
Abstract
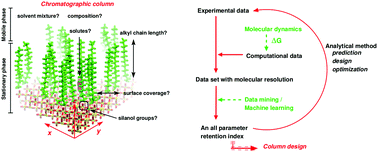
The dynamics of solvated molecules at the solid/liquid interface is essential for a molecular-level understanding for the solution thermodynamics in reversed phase liquid chromatography (RPLC). The heterogeneous nature of the systems and the competing intermolecular interactions makes solute retention in RPLC a surprisingly challenging problem which benefits greatly from modelling at atomistic resolution. However, the quality of the underlying computational model needs to be sufficiently accurate to provide a realistic description of the energetics and dynamics of systems, especially for solution-phase simulations. Here, the retention thermodynamics and the retention mechanism of a range of benzene-derivatives in C18 stationary-phase chains in contact with water/methanol mixtures is studied using point charge (PC) and multipole (MTP) electrostatic models. The results demonstrate that free energy simulations with a faithful MTP representation of the computational model provide quantitative and molecular level insight into the thermodynamics of adsorption/desorption in chromatographic systems while a conventional PC representation fails in doing so. This provides a rational basis to develop more quantitative and validated models for the optimization of separation systems.


 Please wait while we load your content...
Please wait while we load your content...