Catalytic promiscuity of the non-native FPP substrate in the TEAS enzyme: non-negligible flexibility of the carbocation intermediate†
Abstract
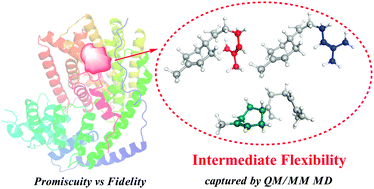
The TEAS, one of the sesquiterpene cyclases (FPPC), shows enzyme promiscuity that can effectively catalyze both the natural substrate (trans,trans)-FPP and the non-native (cis,trans)-FPP substrate to generate diverse products/byproducts. So far, the catalytic mechanism of the promiscuous substrate is still unclear. In this work, QM(DFT)/MM MD simulations were employed to illuminate the predominant 1,6-closure pathway reaction mechanism for the non-native substrate (cis,trans)-FPP, while the 1,10-closure pathway is the major reaction for the native substrate. It has been revealed that the catalytic promiscuity of TEAS is mostly attributable to the notable conformational dynamics of the branching intermediate bisabolyl cation. The comparative studies to FSTS (another widely studied FPPC) further indicate that the intrinsic intermediate flexibility in TEAS is highly correlated to the plasticity of the enzyme active site pocket contour. Finally, we propose a general picture for controlling the promiscuity and fidelity in FPPC catalysis, including substrate folding, intermediate flexibility and key residues.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...