Entropic effects make a more tightly folded conformer of a β-amino acid less stable: UV-UV hole burning and IR dip spectroscopy of l-β3-homotryptophan using a laser desorption supersonic jet technique†
Abstract
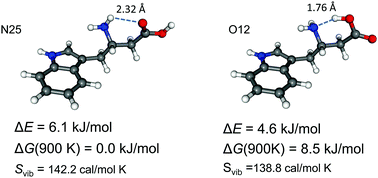
UV-UV hole burning and IR dip spectra of L-β3-homotryptophan were measured by a laser desorption supersonic jet technique as a bottom-up approach to understand the secondary structures of β-peptides. 14 conformers were found by UV-UV hole burning spectroscopy. The conformers were classified into three groups depending on their hydrogen bonding patterns observed in their conformer-specific IR spectra, and tentatively assigned by comparing with quantum chemical calculations. Group 1 had free OH stretch but no NH2 anti-symmetric stretch vibrational transition and was assigned to NH–π hydrogen bonded structures. Group 2, including the most abundant conformer, showed both free OH and NH2 anti-symmetric stretch vibrations, and belonged to NH–O hydrogen bonded conformations. Group 3 of conformers had hydrogen-bonded OH stretch IR transition and had OH–N hydrogen bonds. The internal hydrogen bond of group 3 is a C6 hydrogen bond due to the additional carbon atom at the β position and shows a shorter bond length than that of a C5 hydrogen bond. While the OH–N C6 hydrogen bond is stronger than NH–O, the entropic effect prefers the more flexible NH–O hydrogen bonded structure. It is expected that the unnatural C6 hydrogen bond influences the conformations of β-peptides and builds totally different secondary structures than those of α-peptides.


 Please wait while we load your content...
Please wait while we load your content...