Open-cell voltage and electrical conductivity of a protonic ceramic electrolyte under two chemical potential gradients†
Abstract
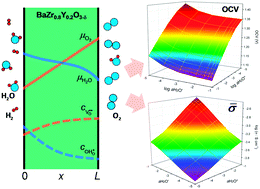
BaZr0.8Y0.2O3−δ, which is a proton-conducting oxide used as an electrolyte for protonic ceramic fuel cells (PCFCs), possesses two mobile ionic charge carriers—oxygen ions and protons—in a crystalline lattice below 500 °C. The equilibrium concentrations of these charge carriers are dependent on water activity. This feature induces a complexity in the distribution of charge carriers within the electrolyte under the influence of the two chemical potential gradients of oxygen and water, which is a typical operating condition in PCFCs. This makes the theoretical derivations of the open-cell voltage and the electrical resistance of the electrolyte difficult. Here, we calculate the distributions of oxygen vacancies and protons across the electrolyte by solving diffusion equations based on the defect chemistry of BaZr0.8Y0.2O3−δ at 500 °C. We then extract the theoretical open-cell voltage and electrical conductivity of the electrolyte in a range of water and oxygen activities that is of interest for PCFCs.


 Please wait while we load your content...
Please wait while we load your content...