Sequence selective photoinduced electron transfer of a pyrene–porphyrin dyad to DNA†
Abstract
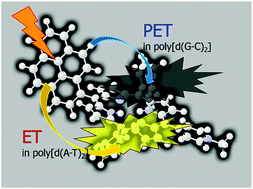
The binding modes of a pyrene–porphyrin dyad, (1-pyrenyl)-tris(N-methyl-p-pyridino)porphyrin (PyTMpyP), to various DNAs (calf thymus DNA (Ct-DNA), poly[d(G–C)2], and poly[d(A–T)2]) have been investigated using circular dichroism and linear dichroism measurements. Based on the polarization spectroscopic results, it can be shown that the pyrenyl and porphryin planes are skewed to a large extent for PyTMPyP in an aqueous environment and in the binding site of poly[d(G–C)2]. In this complex, a photoinduced electron transfer (PET) process between the pyrenyl and porphyrin moieties occurs. On the other hand, PET was not observed in the PyTMPyP–poly[d(A–T)2] complex, whereas the fluorescence intensity of TMPyP was enhanced. The molecular planes of the pyrene and porphyrin moieties are almost parallel in the poly[d(A–T)2] and Ct-DNA adducts. Moreover, the generation of 1O2 species occurs only for the PyTMPyP–Ct-DNA and PyTMPyP–poly[d(A–T)2] complexes. We discuss the photophysical properties of PyTMPyP which are attributed to the binding patterns and the sequence of DNA bases.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...