Controlling factors of oligomerization at the water surface: why is isoprene such a unique VOC?†
Abstract
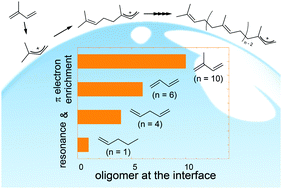
Recent studies have shown that atmospheric particles are sufficiently acidic to enhance the uptake of unsaturated volatile organic compounds (VOCs) by triggering acid-catalyzed oligomerization. Controlling factors of oligomerization at the aqueous surfaces, however, remain to be elucidated. Herein, isoprene (2-methyl-1,3-butadiene, ISO), 1,3-butadiene (1,3-b), 1,4-pentadiene (1,4-p), 1-pentene (1-p), and 2-pentene (2-p) vapors are exposed to an acidic water microjet (1 ≤ pH ≤ 5), where cationic products are generated on its surface within ∼10 μs and directly detected using surface-sensitive mass spectrometry. We found that carbocations form at the air–water interface in all the cases, whereas the extent of oligomerization largely depends on the structure in the following order: ISO ≫ 1,3-b > 1,4-p ≫ 1-p ≈ 2-p. Importantly, the cationic oligomerization of ISO yields a protonated decamer ((ISO)10H+, a C50 species of m/z 681.6), while the pentenes 1-p/2-p remain as protonated monomers. We suggest that ISO oligomerization is uniquely facilitated by (1) the resonance stabilization of (ISO)H+ through the formation of a tertiary carbocation with a conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond pair, and (2) π-electron enrichment induced by the neighboring methyl group. Experiments in D2O and D2O : H2O mixtures revealed that ISO oligomerization on the acidic water surface proceeds via two competitive mechanisms: chain-propagation and proton-exchange reactions. Furthermore, we found that ISO carbocations undergo addition to relatively inert 1-p, generating hitherto uncharacterized co-oligomers.
C bond pair, and (2) π-electron enrichment induced by the neighboring methyl group. Experiments in D2O and D2O : H2O mixtures revealed that ISO oligomerization on the acidic water surface proceeds via two competitive mechanisms: chain-propagation and proton-exchange reactions. Furthermore, we found that ISO carbocations undergo addition to relatively inert 1-p, generating hitherto uncharacterized co-oligomers.


 Please wait while we load your content...
Please wait while we load your content...