Effects of substituents on luminescent efficiency of stable triaryl methyl radicals†
Abstract
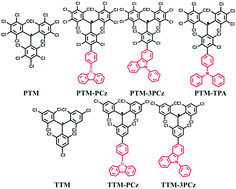
A series of perchlorotriphenyl methyl (PTM) and tris(2,4,6-trichlorophenyl)methyl (TTM) radical derivatives were synthesized. The factors affecting the photoluminescence quantum yields (PLQYs) of π-radicals were studied systematically for the first time through comparing the photophysical properties of the synthesized PTM and TTM radicals. The room-temperature PLQY of a PTM radical derivative achieves to be 56.6%, which is the highest value among the organic near-infrared materials with peak wavelength over 650 nm. The photostabilities of the radicals was significantly enhanced via incorporation of substituent groups. The molecular rigidity, electron donating ability of the donor and dihedral angle between D–A system were found to be the potential factors to affect the luminescent efficiency of the open-shell molecules.


 Please wait while we load your content...
Please wait while we load your content...