The electronic structure and the nature of the chemical bond in CeO2
Abstract
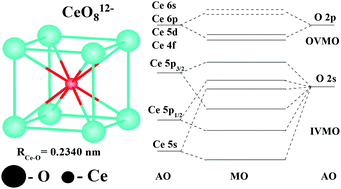
The X-ray photoelectron spectral structure of CeO2 valence electrons in the binding energy range of 0 to ∼50 eV was analyzed. The core-electron spectral structure parameters and the results of relativistic discrete-variational calculations of CeO8 and Ce63O216 clusters were taken into account. Comparison of the valence and the core-electron spectral structures showed that the formation of the inner (IVMO) and the outer (OVMO) valence molecular orbitals contributes to the spectral structure more than the many-body processes. The Ce 4f electrons were established to participate directly in chemical bond formation in CeO2 losing partially their f character. They were found to be localized mostly within the outer valence band. The Ce 5p atomic orbitals were shown to participate in the formation of both the inner and the outer valence molecular orbitals (MOs). A large part in the IVMO formation is taken by the filled Ce 5p1/2, 5p3/2 and O 2s atomic shells, while the Ce 5s electrons participate weakly in the chemical bond formation. The composition and the sequent order of the molecular orbitals in the binding energy range of 0 to ∼50 eV were established. A quantitative scheme for the molecular orbitals of CeO2 was built. This scheme is fundamental for understanding the nature of chemical bonding and also for the interpretation of other X-ray spectra of CeO2. Evaluations revealed that the IVMO electrons weaken the chemical bond formed by the OVMO electrons by 37%.


 Please wait while we load your content...
Please wait while we load your content...