Can 2,2,2-trifluoroethanol be an efficient protein denaturant than methanol and ethanol under thermal stress?†
Abstract
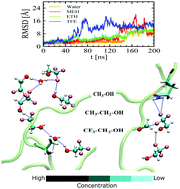
Monohydric alcohols, such as methanol (MEH), ethanol (ETH) and 2,2,2-trifluoroethanol (TFE), have significant effects on biological processes including the protein folding–unfolding phenomenon. Among the several monohydric alcohols, TFE, a fluorine-substituted alcohol, is known to induce a helical structure in proteins. In this work, we report the heterogeneous unfolding phenomenon of a small protein Chymotrypsin Inhibitor 2 in various concentrations of methanol, ethanol and TFE solutions by performing atomistic molecular dynamics simulation studies. Our study reveals that the unfolding phenomenon of CI2 under thermal stress majorly depends on the concentration and the nature of the alcohol. The presence of alcohols in general has been noted to accelerate the unfolding process compared to pure water and TFE, among them all, has been found to speed up the unfolding time scale at low concentrations. The molecular contact frequency between protein and alcohol follows the trend, MEH < ETH < TFE at low concentrations, whereas the trend becomes MEH ∼ ETH > TFE at more concentrated solutions. The differential water-mediated and self-clustering phenomena of alcohols, diverse protein–alcohol hydrogen bond strengths and the concentration dependent restricted inhomogeneous protein–water as well as protein–alcohol hydrogen bond dynamics suggest that TFE, a well known α-helix stabilizer, could be a good competitor among its class of denaturants.


 Please wait while we load your content...
Please wait while we load your content...