Multistep thermal decomposition of granular sodium perborate tetrahydrate: a kinetic approach to complex reactions in solid–gas systems†
Abstract
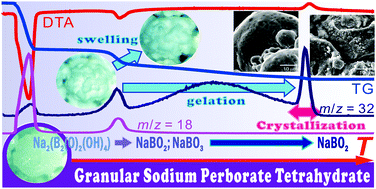
This article demonstrates a kinetic approach to partially overlapping multistep chemical reactions in solid–gas systems as exemplified by the thermal decomposition of granular sodium perborate tetrahydrate. This reaction proceeds via successive thermal dehydration and decomposition occurring at different temperatures to form sodium metaborate. Each reaction process comprises several kinetic steps originating from different physicochemical and physico-geometric phenomena. The partially overlapping multistep processes were characterized using available thermoanalytical techniques and microscopic observations. Conventional isoconversional kinetic analysis and empirical mathematical deconvolution were applied to each reaction process as preliminary kinetic approaches to extracting provable kinetic information. Then, each reaction process was analyzed kinetically based on a cumulative kinetic equation, i.e., kinetic deconvolution analysis. The results of the kinetic deconvolution analysis were further examined by comparison with other kinetic information for the specific kinetic steps obtained from different thermoanalytical measurements. From the results of this comprehensive kinetic approach, the kinetic features of the thermal dehydration and decomposition processes were revealed by identifying their contributing physicochemical and physico-geometric phenomena and evaluating their influences on the overall multistep processes.


 Please wait while we load your content...
Please wait while we load your content...