Mediating both valence and conduction bands of TiO2 by anionic dopants for visible- and infrared-light photocatalysis†
Abstract
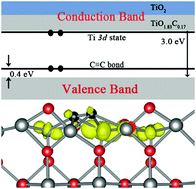
Doping is an effective way to extend the optical absorption of TiO2 to the visible range. Doping of TiO2 by carbon has been found to enhance the water splitting efficiency significantly in experiment. However, the mechanism behind this is elusive. Using the ab initio many-body Green's function theory, we find that the C2 dimer formed on the TiO2 surface produces a shallow delocalized occupied Ti 3d state just below the bottom of the conduction bands. Therefore, band-gap narrowing in carbon-doped TiO2 is caused by the opposite shifts of both valence and conduction bands simultaneously, which is in contrast to the generally accepted idea that anionic dopants can only affect the valence band of TiO2. Optical absorption in the infrared region is also increased compared to reduced TiO2. The spatially well-separated photogenerated electrons and holes might help to reduce the recombination rate of carriers, in favor of improvement in photocatalysis efficiency. This novel behavior of anionic dopants is distinct from previous understandings and may guide the engineering of TiO2.


 Please wait while we load your content...
Please wait while we load your content...