Microsolvation of F−†
Abstract
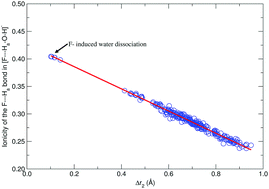
A staggering structural diversity for the microsolvation of F− with up to six water molecules is uncovered in this work. Given the structural variety and the proximity in energy among several local minima, we show here that in order to match available experimental data, statistical averages over contributing structures are needed, rather than assigning experimental values to isolated structures. Our results suggest that the formal charge in F− is strong enough as to induce partial and total dissociation of water molecules and to alter the nature of the surrounding network of water to water hydrogen bonds. We provide an extensive analysis of bonding interactions under the NBO and QTAIM formalisms, our main results suggest a complex interplay between ionic and covalent characters for the F⋯H interactions as a function of the separation between the atoms.


 Please wait while we load your content...
Please wait while we load your content...