Application of far-infrared spectroscopy to the structural identification of protein materials
Abstract
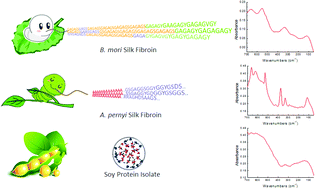
Although far-infrared (IR) spectroscopy has been shown to be a powerful tool to determine peptide structure and to detect structural transitions in peptides, it has been overlooked in the characterization of proteins. Herein, we used far-IR spectroscopy to monitor the structure of four abundant non-bioactive proteins, namely, soybean protein isolate (SPI), pea protein isolate (PPI) and two types of silk fibroins (SFs), domestic Bombyx mori and wild Antheraea pernyi. The two globular proteins SPI and PPI result in broad and weak far-IR bands (between 50 and 700 cm−1), in agreement with those of some other bioactive globular proteins previously studied (lysozyme, myoglobin, hemoglobin, etc.) that generally only have random amino acid sequences. Interestingly, the two SFs, which are characterized by a structure composed of highly repetitive motifs, show several sharp far-IR characteristic absorption peaks. Moreover, some of these characteristic peaks (such as the peaks at 260 and 428 cm−1 in B. mori, and the peaks at 245 and 448 cm−1 in A. pernyi) are sensitive to conformational changes; hence, they can be directly used to monitor conformational transitions in SFs. Furthermore, since SF absorption bands clearly differ from those of globular proteins and different SFs even show distinct adsorption bands, far-IR spectroscopy can be applied to distinguish and determine the specific SF component within protein blends.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...