Intra-cavity proton bonding and anharmonicity in the anionophore cyclen†
Abstract
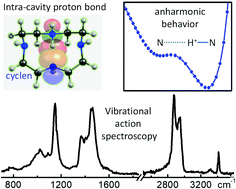
Proton bonding drives the supramolecular chemistry of a broad range of materials with polar moieties. Proton delocalization and electronic charge redistribution have a profound impact on the structure of proton-bound molecular frameworks, and pose fundamental challenges to quantum chemical modelling. This study provides insights into the structural and spectral signatures of the intramolecular proton bond formed in a benchmark polyazamacrocycle anionophore (cyclen, 1,4,7,10-tetraazacyclododecane). Infrared action spectroscopy is employed to characterize the macrocycle, isolated in protonated form. In its most stable configuration, protonated cyclen adopts an open arrangement of Cs symmetry with a particularly strong NHδ+⋯N bond across the cavity. The quantum chemical analysis of the infrared spectrum reveals intrinsic difficulties for the accurate description of the vibrational modes of the system. The reconciliation of the computational predictions with experiment demands a careful anharmonic treatment of the proton motion, which exposes the limitations of current methods. Best results are obtained with the incorporation of anharmonicity only to the fundamental modes directly related to motions of the proton. However, the full anharmonic treatment of the system fails to describe correctly the vibrations related to the macrocycle backbone. The results should serve as motivation for new developments in the modelling of proton bonded systems.


 Please wait while we load your content...
Please wait while we load your content...