Reductive defluorination of graphite monofluoride by weak, non-nucleophilic reductants reveals low-lying electron-accepting sites†
Abstract
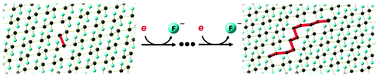
Graphite monofluoride (GF) can undergo reductive defluorination in the presence of weak, non-nucleophilic reductants. This leads to a new approach to GF–polyaniline composites as cathode materials for significantly improving the discharge capacity of primary lithium batteries. We postulate that the reduction is mediated by residual π-bonds in GF.


 Please wait while we load your content...
Please wait while we load your content...
