A first-principles study of stability of surface confined mixed metal oxides with corundum structure (Fe2O3, Cr2O3, V2O3)†
Abstract
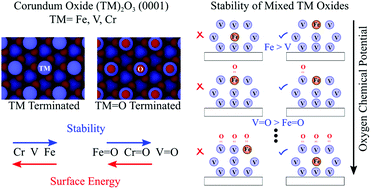
Surface-confined mixed metal oxides can have different chemical properties compared to their host metal oxide support. For this reason, mixed transition metal oxides can offer tunable redox properties. Herein, we use density functional theory to predict the stability of the (0001) surface termination for mixed metal oxides consisting of Fe2O3, Cr2O3 and V2O3. We show that the pure oxide surface stability can predict the surface segregation preference of the surface-confined mixed metal oxides. We focus on substitution of Fe in the V2O3(0001) surface, for which we observe that Fe substitution increases the reducibility of the resulting mixed metal oxide surface. Our results suggest Fe is only stable on the surface under very high temperature and/or low-pressure conditions. Using thermodynamic relationships, we predict the transition points for these surface-confined mixed metal oxides at which exchange between surface/subsurface and subsurface/surface metal atoms occur due to changes in the oxygen chemical potential.


 Please wait while we load your content...
Please wait while we load your content...