Evaporation of nanoscale water on a uniformly complete wetting surface at different temperatures†
Abstract
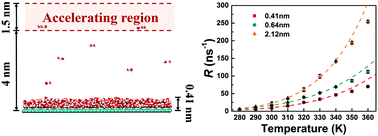
The evaporation of nanoscale water films on surfaces affects many processes in nature and industry. Using molecular dynamics (MD) simulations, we show the evaporation of a nanoscale water film on a uniformly complete wetting surface at different temperatures. With the increase in temperature, the growth of the water evaporation rate becomes slow. Analyses show that the hydrogen bond (H-bond) lifetimes and orientational autocorrelation times of the outermost water film decrease slowly with the increase in temperature. Compared to a thicker water film, the H-bond lifetimes and orientational autocorrelation times of a monolayer water film are much slower. This suggests that the lower evaporation rate of the monolayer water film on a uniformly complete wetting surface may be caused by the constriction of the water rotation due to the substrate. This finding may be helpful for controlling nanoscale water evaporation within a certain range of temperatures.


 Please wait while we load your content...
Please wait while we load your content...